Developing a Next Generation SARS-CoV-2 Vaccine
23 Mar 2021|Jack Tan
- Research
We have been battling the SARS-CoV-2 pandemic for over a year. During this time, we have made huge progress towards developing vaccines and drugs to prevent and cure infections. However, new viral variants are being discovered (eg. South African variant, UK variant, etc) which present new challenges and may reduce vaccine effectiveness. To tackle these new variants, the next generation of vaccines is needed and my colleagues and I are working on exactly this.
SARS-CoV-2 pandemic
2020 has been a year like no other with the SARS-CoV-2 pandemic and the development of Covid-19 vaccines in record time. Coronaviruses have been around for decades, but they were not a top priority among scientists for vaccine development as it seemed they caused nothing but a mild cold. It was not until 2002 when a novel coronavirus (SARS-CoV) caused a pandemic in Asia with a 10% mortality rate that scientists realised the importance of a vaccine against coronaviruses. The efforts of vaccine development against SARS-CoV halted eventually as the pandemic was wiped out by quarantines and other non-vaccine based interventions. Nevertheless, all the previous research on a SARS-CoV vaccine has laid the foundations for the development of SARS-CoV-2 vaccines. At the time of writing, 2 SARS-CoV-2 vaccines (Pfizer and Oxford/AstraZeneca) have been approved by the MHRA for emergency use in the UK and around 20M people have received their first vaccine dose. So once we’re all vaccinated is that the end for Sars-Cov-2?
Same virus different variants
Sadly, no. Virologists, like me, have been worrying about the emergence of SARS-CoV-2 variants which will allow it to continue to infect humans. Similar to flu, which has been circulating in the human population for more than 100 years, SARS-CoV-2 will mutate, and new variants will arise. Some of these new variants will allow the virus to “escape” the immunity acquired from previous infection or vaccination. Several recently published studies have shown that people infected with the original virus, or immunised with the current vaccines, might have reduced protection against these new variants, especially the B.1.351 (South African) variant.
On top of this, we also know that there will be more coronavirus outbreaks in the future when a new coronavirus jumps from animal reservoirs to humans. We therefore need better approaches to tackle the emerging variants and any novel coronaviruses in the future, ideally pre-pandemic.
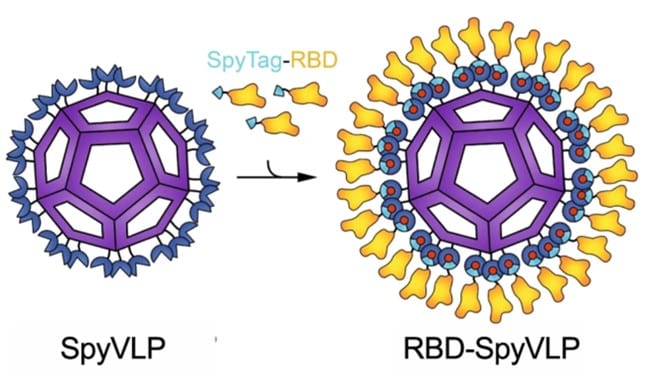
Next generation pan-coronavirus vaccine
Since February 2020, my colleagues and I have been working on a potential vaccine that can overcome the weaknesses that the current vaccines have. Our vaccine, namely, RBD-SpyVLP (Tan et. al., 2020, Nat Comms), has a nanoparticle core (called a viral like particle (VLP)) which we decorate on the outside with the Receptor Binding Domain (RBD) of SARS-CoV-2. RBD is responsible for the virus being able to bind to and enter human cells so by decorating our core with RBD we can elicit an immune response that can prevent viral infection. We decorate the VLP core using a protein glue called SpyCatcher creating a SpyVLP (image below). SpyCatcher sticks incredibly strongly to its partner SpyTag which we attach onto the RBD creating a SpyTag-RBD. Now when we mix the sticky SpyVLP core with the SpyTag-RBD the SpyVLP gets covered in RBD, much like rolling a ball of dough through flour. This SpyCatcher/SpyTag system was developed by Mark Howarth in Biochemistry and was originated from the bacteria Streptococcus pyogenes.
The road less travelled
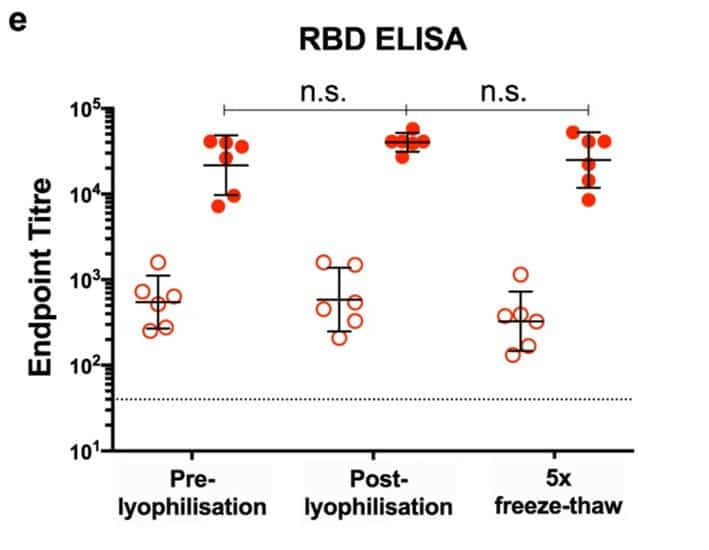
A problem many vaccines face is getting from a lab like ours, where they are made, into patients who could be thousands of miles away. One of the issues is that vaccines can degrade and go off over time just like food. Similarly to food, the solution is putting it in the fridge or, better yet, freezer. I have shown that our vaccine is very thermostable meaning it remains good even when left out at room temperature, or repeatedly frozen and thawed or freeze dried (lyophilized) and reconstituted.
As you can see in the graph, lyophilised RBD-SpyVLP and RBD-SpyVLP that had undergone 5x freeze-thaw cycles are equally good at eliciting immunity as freshly made (pre-lyophilised) RBD-SpyVLP in mice (open circles: after 1 dose, closed circles: after 2 doses). This thermostability makes the RBD-SpyVLP a very attractive vaccine to distribute globally especially in countries with limited cold chains where other vaccines may go bad before ever reaching their destinations.
My research group and I believe that the RBD-SpyVLP is a vaccine with huge potential. Its ability to raise immunity against many different variants as well as its’ hardiness are keys to ensuring we have vaccines for future variants and novel coronaviruses, accessible in every corner of the globe. We are currently raising funds to support the production of the RBD-SpyVLP in preparation for human trials and are excited to take the next steps in development.
Category: Research
